SrF2
Strontium Fluoride (SrF2) is one of the most important alkaline-earth fluorides. It has many physical properties, such as low energy phonon, high ionization, high resistance coefficient and good anion conductivity. It has been widely used in light-emitting devices, optical imaging, biomarkers, anion conductors and other fields. Compared with oxide and calcium fluoride, strontium fluoride has lower phonon energy, higher thermal conductivity and negative thermal optical coefficient, especially negative thermal optical coefficient, which can compensate the thermal lens effect in the process of laser oscillation. Strontium fluoride has promoted the research of optical materials and the improvement of optical instruments with its unique material structure and properties
Parameter
| Crystal Structure | Single crystal, synthetic |
| Crystal Type | Cubic (CaF2 type) |
| Lattice Constants | 5.7996 |
| Density | 4.24 g/cm3 |
| Melting Point | 1477°C |
| Thermal Conductivity /(W·m-1·K-1@298k) | 1.42 |
| Specific Heat Capacity/ (J·kg-1·K-1) | 544 |
| Thermal Expansion /(10-6·K-1@293k ) | 18.4 |
| Hardness (Mohs) | 130 |
| Young`s Modulus /(GPa) | 99.91 |
| Shear Modulus /(GPa) | 34.6 |
| Bulk Modulus /(GPa) | 24.65 |
| Elastic Coefficient/(GPa) | C11=124; C12=44; C44= 31.8 |
| Cleavage | -111 |
| Solubility in Water/(g/100g) | 0.021@298K |
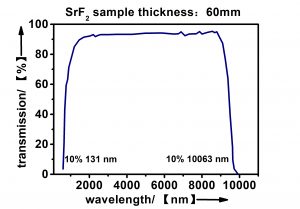
| Transmission Range (50%) | 0.14 … 9µm @thickness 2mm |
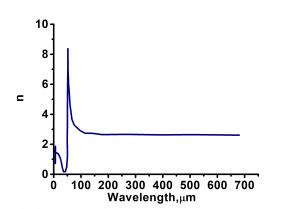
| Refractive Index | 1.436914@633nm |
| Reflective Loss | 1.74% @10 µm |
| Poisson Ratio | 0.29 |
| Dielectric Constant | 7.69@2MHz |
| Reststrahlen | 40µm |
- Poorly soluble in water, ethanol and methanol
- High conductivity and optical properties similar to calcium fluoride
- Can transmit ultraviolet and infrared waves
- Very dangerous to the health, poisonous when inhaled or ingested and in low concentration can cause irritation to skin and eyes.
- Not flammable
- UV and VUV spectroscopy
- Manufacture optical materials for different devices, like glasses, windows and lenses
- Intermediate infrared laser matrix material
| [1] Yagoub M , Swart H C , Coetsee E . Effect of Yb 3+ ions on Structural and NIR emission of SrF 2 :Eu 2+ /Pr 3+ down-conversion containing Na + ions[J]. Materials Research Bulletin, 2017, 93:170-176. |
| [2] Karimi M , Hesaraki S , Nezafati N . In vitro biodegradability–bioactivity–biocompatibility and antibacterial properties of SrF 2 nanoparticles synthesized by one-pot and eco-friendly method based on ternary strontium chloride-choline chloride-water deep eutectic system[J]. Ceramics International, 2018:12877-12885. |
| [3] Thermoluminescence dosimetry features of DY and Cu doped SrF2 nanoparticles under gamma irradiation[J]. Applied Radiation & Isotopes, 2015, 105:ARID1500488. |
| [4] Gao Y , Mei B , Li W , et al. Effect of Yb3+ concentration on microstructure and optical properties of Yb: SrF2 transparent ceramics[J]. Optical Materials, 2020, 105:109869. |
| [5] 李建刚, 何向明, 赵如松. Electrochemical performance of SrF2-coated LiMn2O4 cathode material for Li-ion batteries[J]. Transactions of Nonferrous Metals Society of China, 2007(06):205-208. |
| [6] Biroon M K , Zahedifar M , Sadeghi E , et al. Preparation, kinetic analysis and thermoluminescent dosimetry features of highly sensitive SrF2:Dy phosphor[J]. Radiation Physics and Chemistry, 2019, 159:1-5. |
| [7] Hesaraki S , Karimi M , Nezafati N . The synergistic effects of SrF2 nanoparticles, YSZ nanoparticles, and poly-ε-l-lysin on physicomechanical, ion release, and antibacterial-cellular behavior of the flowable dental composites – ScienceDirect[J]. Materials Science and Engineering: C, 109. |
| [8] Ji G , Hong G J , Bae C H , et al. Infrared-laser precipitation of Dy3+-Yb3+ codoped SrF2 nanocrystals in glass and upconversion luminescence[J]. Applied Surface Science, 2019, 478:412-416. |
| [9] Yagoub M , Swart H C , Kroon R E , et al. Low temperature photoluminescence study of Ce 3+ and Eu 2+ ions doped SrF 2 nanocrystals[J]. Physica B Condensed Matter, 2017:S0921452617304891. |
| [10] Zhou Z , Li W , J Song, et al. Application of Judd-Ofelt theory in analyzing Nd3+ doped SrF2 and CaF2 transparent ceramics[J]. Journal of the European Ceramic Society, 2019. |
| [11] Scintillation Properties of SrF 2 Translucent Ceramics and Crystal[J]. Optik, 2018:S0030402618305758. |
| [12] Chuklina N , Mysovsky A . Theoretical study of self-trapped hole diffusion in CaF2, SrF2, BaF2 crystals[J]. Radiation Measurements, 2019, 128:106135-. |
| [13] [ Farha Khatun, Thakur P , Hoque N A , et al. In situ synthesized SrF2/polyvinylidene fluoride nanocomposite film based photo-power cell with imperious performance and stability[J]. Electrochimica Acta, 2018. |
| [14] Hosoya H , Arita M , Hamada K , et al. Epitaxial growth of Fe nanodots on SrF 2/Si (111)[J]. Materials Science and Engineering C, 2006, 26(5):1146-1150. |
| [15] Yanagida T , Fujimoto Y , Fukuda K , et al. Ce-doped LiF-SrF2 eutectic scintillators for thermal neutron detection produced at different solidification rates[J]. Optical Materials, 2013, 35(7):1449-1454. |
| [16] Ye J , Qin W , Zhang J . Preparation and optical properties of SrF2 : Eu3+ nanospheres[J]. Journal of Fluorine Chemistry, 2008, 129(6):515-518. |
| [17] Zhou Z , Li W , Song J , et al. Synthesis and characterization of Nd 3+ doped SrF 2 nanoparticles prepared by precipitation method[J]. Ceramics International, 2017, 44(4). |
| [18] Thomas M E . Strontium Fluoride (SrF2)[J]. Handbook of Optical Constants of Solids, 1997. |
| [19] Lewandowski, Tomasz, Walas, et al. Eu3+ doped tellurite glass ceramics containing SrF2 nanocrystals: Preparation, structure and luminescence properties[J]. Journal of Alloys and Compounds: An Interdisciplinary Journal of Materials Science and Solid-state Chemistry and Physics, 2017. |
| [20] Jia S , Li C , Zhao Z , et al. Er 3+ -doped ZnF 2 -BaF 2 -SrF 2 -YF 3 fluoride glasses for 2.7 μm laser applications[J]. Materials Letters, 2018, 227. |
| [21] Yagoub M , Swart H C , Dhlamini M S , et al. Near infrared quantum cutting of Na+ and Eu2+-Yb3+ couple activated SrF2 crystal[J]. Optical Materials, 2016, 60:521-525. |
| [22] A C R K , B C S D V , A S K , et al. Enhanced visible emissions of Pr 3+ -doped oxyfluoride transparent glass-ceramics containing SrF 2 nanocrystals[J]. Ceramics International, 2018, 44( 2):1737-1743. |
| [23] Demkiv T M , Halyatkin O O , Vistovskyy V V , et al. X-ray excited luminescence of polystyrene composites loaded with SrF_2 nanoparticles[J]. Nuclear Instruments & Methods in Physics Research. Section A, Accelerators, Spectrometers, Detectors and Associated Equipment, 2017, 847(mar.1):47-51. |
| [24] Deng, Dong, YY, et al. Revealing the influences of cellulose on cellulose/SrF2 nanocomposites synthesized by microwave-assisted method[J]. IND CROP PROD, 2016, 2016,85(-):258-265. |
| [25] Balabhadra S , Reid M F , V Golovko, et al. Absorption Spectra, Defect Site Distribution and Upconversion Excitation Spectra of CaF$_2$/SrF$_2$/BaF$_2$:Yb$^{3+}$:Er$^{3+}$ Nanoparticles[J]. 2019. |
| [26] Im A , Yw A , Ez B . The effect of Gd3+ doping on luminescence properties of (Gd, Ce): SrF2 nanopowders and transparent ceramics – ScienceDirect[J]. Journal of Luminescence, 224. |
| [27] Przybylska D , Grzyb T . Synthesis and up-conversion of core/shell SrF 2 :Yb 3+, Er 3+ @SrF 2 :Yb 3+, Nd 3+ nanoparticles under 808, 975, and 1532nm excitation wavelengths[J]. Journal of Alloys and Compounds, 831. |
| [28] Gulina L B , Sch?Fer M , Privalov A F , et al. Synthesis and NMR investigation of 2D nanocrystals of the LaF3 doped by SrF2[J]. Journal of Fluorine Chemistry, 2016, 188:185-190. |
| [29] Yagoub M , Swart H C , Coetsee E . Concentration quenching, surface and spectral analyses of SrF2:Pr3+ prepared by different synthesis techniques[J]. Optical Materials, 2015, 42:204-209. |
| [30] Enhanced electrochemical performance of SrF 2 -modified Li 4 Ti 5 O 12 composite anode materials for lithium-ion batteries |




Leave a Reply