MgF2
Magnesium Fluoride (MgF2) is commonly used for UV windows, lenses and polarisers. It is also useful in its transmission range for some IR spectroscopy applications. Magnesium Fluoride (MgF2) Windows offer excellent broadband transmission from the deep-UV to the mid-infrared. DUV transmission makes them ideal for use at the Hydrogen Lyman-alpha line and for UV radiation sources and receivers, as well as excimer laser applications. Windows, lenses, and prisms made of this material can be used over the entire range of wavelengths from 0.120 μm (vacuum ultraviolet) to 8.0 μm (infrared). MgF2 is tough and works and polishes well, but it is slightly birefringent and should be cut with the optic axis perpendicular to the plane of the window or lens, Generally, Magnesium Fluoride for laser use is recommended to be oriented along the optic axis to avoid birefringent effects. It is particularly useful for excimer laser application.
Parameter
| Orientation | [100] or [001] < ±0.5° |
| Orientation Tolerance | < 0.5° |
| Parallelism | 5〞 |
| Perpendicularity | 3ˊ |
| Surface Quality | 10-5 (Scratch/Dig) |
| Wavefront Distortion | <λ/4@632 nm |
| Surface Flatness | <λ/8 @632 nm |
| Clear Aperture | >90% |
| Chamfer | <0.1×45° |
| Thickness/Diameter Tolerance | ±0.05 mm |
| Maximum Dimensions | Dia150 mm×60mm(C-cut) |
| Crystal Structure | tetragonal |
| Lattice Constants | 4.64 |
| Density | 3.18 g/cm3 |
| Melting Point | 1255°C |
| Thermal Conductivity /(W·m-1·K-1@25°C) | 0.3 |
| Specific Heat/ (J·g-1·K-1) | 1.003 |
| Thermal Expansion /(10-6·K-1@25°C ) | 13.7 |
| Hardness (Mohs) | 4.15 |
| Young`s Modulus /GPa | 138.5 |
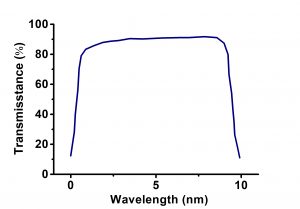
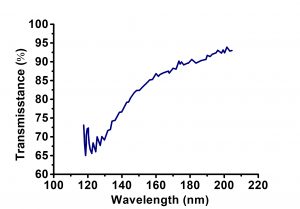
| Transmission Range | 0.11 … 7.5 µm |
| Refractive Index | no = 1.3836, ne = 1.3957 @0.405 µm |
| Reflective Loss | 5.1% @4.0 µm; 11.2% @0.12 µm |
| Poisson Ratio | 0.271 |
| μm | No | Ne | μm | No | Ne | μm | No | Ne |
| 0.1137 | 1.7805 | 0.1149 | 1.742 | 0.1179 | 1.68 | |||
| 0.1198 | 1.651 | 0.121 | 1.628 | 1.632 | 0.13 | 1.566 | 1.568 | |
| 0.14 | 1.5095 | 1.523 | 0.15 | 1.48 | 1.494 | 0.16 | 1.461 | 1.475 |
| 0.17 | 1.448 | 1.462 | 0.18 | 1439 | 1.453 | 0.19 | 1.431 | 1.444 |
| 0.2 | 1.423 | 1.437 | 0.22 | 1.413 | 1.426 | 0.248 | 1.403 | 1.416 |
| 0.257 | 1.401 | 1.414 | 0.266 | 1.399 | 1.412 | 0.28 | 1.396 | 1.409 |
| 0.3 | 1.393 | 1.405 | 0.33 | 1.389 | 1.402 | 0.337 | 1.389 | 1.401 |
| 0.35 | 1.387 | 1.4 | 0.355 | 1.386 | 1.399 | 0.4 | 1.384 | 1.396 |
| 0.546 | 1.379 | 1.39 | 0.7 | 1.376 | 1.388 | 1.087 | 1.373 | 1.385 |
| 1.512 | 1.37 | 1.382 | 2 | 1.368 | 1.379 | 2.5 | 1.364 | 1.375 |
| 3.03 | 1.36 | 1.37 | 3.571 | 1.354 | 1.364 | 4 | 1.349 | 1.359 |
| 4.546 | 1.341 | 1.35 | 5 | 1.334 | 1.343 | 5.556 | 1.324 | 1.332 |
- Excellent transmission from 120nm to 7um
- Chemically stable
- Large resistance to thermal shock
- Irradiation does not lead to color centers
- Uniformly distributed Co
- Window and focusing mirror for deep uv and excimer lasers
- Optical fiber communication,
- Wave plate,High Energy WavePlate
- Achromatic Waveplates
- Glanprisms
| [1] Ning Y , Wang J , Ou C , et al. NiCr–MgF2 spectrally selective solar absorber with ultra-high solar absorptance and low thermal emittance[J]. Solar Energy Materials and Solar Cells, 2019, 206:110219. |
| [2] Matsuo T , Kato T , Kimura H , et al. Evaluation of dosimetric properties of Tb-doped MgF2 transparent ceramics[J]. Optik – International Journal for Light and Electron Optics, 2019, 203:163965. |
| [3] Hughes-Currie R B , Ivanovskikh K V , Wells J , et al. Intrinsic electronic excitations and impurity luminescent centres in NaMgF_3 and MgF_2 doped with Yb~(2+)[J]. Optical Materials, 2020, 99(Jan.):109553.1-109553.7. |
| [4] Song J D , Song T Y , Zhang T T , et al. High performance V 2 O 5 /MgF 2 catalysts for gas-phase dehydrofluorination of 1,1,1,3,3-pentafluoropropane: Support-induced evolution of new active sites[J]. Journal of Catalysis, 2018, 364:271-281. |
| [5] Arroussi, A, Ghezali, et al. First-principles study of the structural, electronic and optical properties of MgF2[J]. Journal for Light and Electronoptic, 2018. |
| [6] Fang X X , Liao W M , Song J D , et al. Effect of Fe promotion on the performance of V2O5/MgF2 catalysts for gas-phase dehydrofluorination of 1,1,1,3,3-pentafluoropropane[J]. Applied Surface Science, 2019, 490(OCT.1):365-371. |
| [7] Sun X , Tu J , Li L , et al. Preparation of wide-angle and abrasion-resistant multi-layer antireflective coatings by MgF 2 and SiO 2 mixed sol[J]. Colloids and Surfaces A Physicochemical and Engineering Aspects, 2020:125106. |
| [8] Toghyani S , Khodaei M , Razavi M . Magnesium scaffolds with two novel biomimetic designs and MgF2 coating for bone tissue engineering[J]. Surface and Coatings Technology, 2020, 395:125929. |
| [9] Syed W , Rafiq N , Ali A , et al. Multilayer AR coatings of TiO 2 /MgF 2 for application in optoelectronic devices[J]. Optik – International Journal for Light and Electron Optics, 2017, 136:564-572. |
| [10] Nakamura F , Kato T , Okada G , et al. Scintillation, dosimeter and optical properties of MgF 2 transparent ceramics doped with Gd 3+[J]. Materials Research Bulletin, 2017, 98. |
| [11] Chen H , Jie Y , Chang L , et al. Reaction behavior of MgF2 powder in hexafluoropropylene/air atmospheres at high temperatures[J]. Solid State Ionics, 2019, 340:115016. |
| [12] Ding K , Zhang X , Ning L , et al. Hue Tunable, High Color Saturation and High-Efficiency Graphene/Silicon Heterojunction Solar Cells with MgF 2 /ZnS Double Anti-Reflection Layer[J]. Nano Energy, 2018:S2211285518300673. |
| [13] Li J , Xu W , Lin X , et al. A Ca-deficientca-deficient hydroxyapatite (CDHA)/MgF 2 bi-layer coating with unique nano-scale topography on biodegradable high-purity Mg[J]. Colloids and Surfaces B: Biointerfaces, 2020, 190:110911. |
| [14] JS Youn, DT Phan, CM Park,等. Enhancement of hydrogen sorption properties of MgH2 with a MgF2 catalyst[J]. International Journal of Hydrogen Energy, 2017. |
| [15] Sun X , Xu X , Song G , et al. Preparation of MgF2/SiO2 coating with broadband antireflective coating by using sol–gel combined with electron beam evaporation[J]. Optical Materials, 2020, 101:109739-. |
| [16] Mohammad, Reza, Tohidifar. Low-temperature sintering and densification kinetics of sol-gel derived lithium-mica glass-ceramics through MgF2 addition – ScienceDirect[J]. Journal of Non-Crystalline Solids, 521:119497-119497. |
| [17] Reuna J , Polojarvi V , Paakkonen P , et al. Influence of ex-situ annealing on the properties of MgF2 thin films deposited by electron beam evaporation[J]. Optical Materials, 2019, 96:109326-. |
| [18] Park H W , Jo H , Anoop G , et al. Transition metal ion Co-Doped MgO–MgF2-GeO2:Mn4+ red phosphors for white LEDs with wider color reproduction gamut[J]. Journal of Alloys and Compounds, 2019, 818:152914. |
| [19] Microstructure, mechanical properties and sintering mechanism of pressureless-sintered porous Si3N4 ceramics with YbF3-MgF2 composite sintering aids[J]. Ceramics International, 2020, 46(2):2558-2564. |
| [20] Fujihara S , Tada M , Kimura T . Preparation and characterization of MgF2 thin film by a trifluoroacetic acid method[J]. Journal of Nano Research, 2008, 304(1):550. |
| [21] Ji Z , Bao L , Wang H , et al. Preparation of super-hydrophobic antireflective films by rod-like MgF 2 and SiO 2 mixed sol[J]. Materials Letters, 2017, 207:21-24. |
| [22] Nakamura F , Kato T , Okada G , et al. Scintillation and storage luminescence properties of MgF 2 transparent ceramics doped with Ce 3+[J]. Optical Materials, 2017, 72:470-475. |
| [23] Fumiya, Nakamura, Takumi, et al. Scintillation and TSL properties of MgF2 transparent ceramics doped with Eu2+ synthesized by spark plasma sintering[J]. Journal of Alloys & Compounds, 2017. |
| [24] Jia Z , Wei M , Bai Y , et al. Hollow nano- MgF 2 supported catalysts: Highly active and stable in gas-phase dehydrofluorination of 1,1,1,3,3-pentafluoropropane[J]. Applied Catalysis B Environmental, 2018, 238:599-608. |
| [25] Korenko M , Šimko, Fratišek, J Mlynáriková, et al. Physico – chemical properties of (MgF2 – CaF2 – (LiF))eut – MgO system as a molten electrolyte for Mg electrowinning[J]. Journal of Molecular Liquids, 2018, 275:535-543. |
| [26] Cotter T M , Thomas M E , Tropf W J . Magnesium Fluoride (MgF2)[J]. handbook of optical constants of solids, 1998. |
| [27] Chen H , Li C , Jie Y . Reaction kinetics of MgF 2 powder in air at high temperature[J]. Corrosion science, 2017, 126:121-126. |
| [28] Wang Y , Wang S , Deng S , et al. Effect of the addition of MgF 2 and NaF on the thermal, optical and magnetic properties of fluoride glasses for sensing applications[J]. Optical Materials, 2017, 72:341-345. |
| [29] Sharma, Anuj K . Simulation and analysis of Au-MgF 2 structure in plasmonic sensor in near infrared spectral region[J]. Optics & Laser Technology, 2018, 101:491-498. |
| [30] Ab initio calculations of pure and Co+2-doped MgF2 crystals |





Leave a Reply