KBr
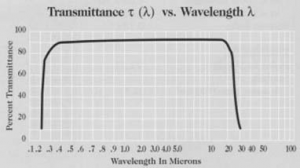
Potassium Bromide (KBr) crystal is water-souble, and it is easily deliquescent. That cannot be chemically polished. It has a high transmittance and a wide pass band, so it’s commonly used for transmission windows, FTIR spectrophotometers and beam splitters for spectrophotometers. The KBr substarte and windows are soft and Hygroscopic. KBr single crystal uniform texture and transparent, has a high infrared transmittance, the transmittance greater than 90% when thickness less 10mm and in the range of 4000-500cm-1, no impurity absorption. KCI single crystal as a laser window material, the optical performance is excellent.
Parameter
| Orientation | <100>, <110>, <111> |
| Orientation Tolerance | < 0.5° |
| Parallelism | 5〞 |
| Perpendicularity | 3ˊ |
| Surface Quality | 10-5 (Scratch/Dig) |
| Wavefront Distortion | <λ/4@632 nm |
| Surface Flatness | <λ/8 @632 nm |
| Clear Aperture | >90% |
| Chamfer | <0.1×45° |
| Thickness/Diameter Tolerance | ±0.05 mm |
| Crystal Structure | Cubic |
| Symmetry Class | m3m |
| Lattice Constants | 6.598 |
| Specific Mass | 2.75 g/cm3 |
| Melting Point | 728°C |
| Cleavability | (100), perfect |
| Thermal Conductivity /(W·m-1·K-1@46°C) | 4.81 |
| Specific Heat (J·kg-1·K-1) | 452.2 |
| Thermal Expansion(10-6·K-1@60°C) | 36.6 …39.6 |
| Hardness (Mohs) | 1.5 |
| Vickers Microhardness(GPa) | 0.1 |
| Constant of Elastic Compliance(10-12·Pa-1) | S11=30.29, S12=-4.18, S44=194.92 |
| Young Modulus (GPa) | 33.0@<100>, 13.8@<111> |
| Shear Modulus (GPa) | 9.0@<100>, 5.1@<111> |
| Transmission Range | 0.21 … 28.0µm |
| Refractive Index | 1.5251@10.6µm |
| Thermo-optic Coefficient(10-6·K-1@60°C,3.4µm) | -0.395 … -0.429 |
| Poisson Ratio | 0.138 |
| λ(μm) | n | λ(μm) | n | λ(μm) | n |
| 0.2 | 2.0995 | 5 | 1.5334 | 11 | 1.5217 |
| 0.5 | 1.57 | 6 | 1.5319 | 12 | 1.5204 |
| 1 | 1.5444 | 7 | 1.5303 | 15 | 1.4403 |
| 2 | 1.5383 | 8 | 1.5285 | 20 | 1.3822 |
| 3 | 1.5357 | 9 | 1.5265 | 30 | 1.0912 |
| 4 | 1.5346 | 10 | 1.5242 | ||
Feature
Application
Reference
Feature
- Wide-band good conductor
- Water-soluble, easy to deliquesce and cannot be chemically polished
- Can grow epitaxial films on a featureless substrate
- Good resistance to mechanical shock
Application
- Substrate for Epitaxial growth
- Be used in the production of infrared spectroscopy analyzer
- Ultraviolet and infrared optical components
- Prisms, lenses, filters and various laser windows, infrared devices, optical, laser crystal instruments
Reference
| [1] Ai Z , Li S , Zhao Y , et al. Atomic insights into flotation separation of KCl and NaCl from a new viewpoint of hydration layer: A molecular dynamic study[J]. Colloids and Surfaces A Physicochemical and Engineering Aspects, 2020, 602:125071. |
| [2] Hu J , Luo G , Li Z , et al. Deactivation mechanism of KCl and K2SO4 poisoned V2O5/WO3-TiO2 catalyst on gaseous elemental mercury oxidation[J]. Fuel, 2020, 278(9):118245. |
| [3] Huang Y , Liu H , Yuan H , et al. Migration and speciation transformation of K and Cl caused by interaction of KCl with organics during devolatilization of KCl-loaded model biomass compounds[J]. Fuel, 2020, 277:118205. |
| [4] Shetty T , Szpunar J A , Faye O , et al. A comparative study of hydrogen generation by reaction of ball milled mixture of magnesium powder with two water-soluble salts (NaCl and KCl) in hot water – ScienceDirect[J]. International Journal of Hydrogen Energy, 2020, 45( 48):25890-25899. |
| [5] Electrochemical behavior of niobium ions in molten KCl-NaCl[J]. Journal of Materials Research and Technology, 2020. |
| [6] Lxwab C , Arl B , Yi Y D , et al. Preparation and FTIR, Raman and SEM characterizations of konjac glucomannan-KCl electrogels – ScienceDirect[J]. Food Chemistry, 2020. |
| [7] Phother-Simon J , Jonsson T , Liske J . Continuous KCl addition in high temperature exposures of 304 L – A way to mimic a boiler environment[J]. Corrosion Science, 2020, 167:108511. |
| [8] Zhang J , Rahman Z U , Wang X , et al. Hot corrosion behaviors of TP347H and HR3C stainless steel with KCl deposit in oxy-biomass combustion[J]. Journal of Environmental Management, 2020, 263:110411-. |
| [9] Zhu T , Hu Ang W , Gong Y . Electrochemical Separation of Uranium from Lanthanide (La, Eu, Gd) Fluorides in Molten LiCl-KCl[J]. Separation and Purification Technology, 2019, 235(12):116227. |
| [10] Abdelfetah, Mounir, Brahim, et al. Determination of water activity, osmotic coefficient, activity coefficient, solubility, excess Gibbs energy and transfer Gibbs energy of KCl-D–sucrose-water mixture at 298.15?K[J]. Journal of Chemical Thermodynamics, 2020, 142:105962-105962. |
| [11] To A , Jy B , Hm A , et al. Fundamental analysis for electrochemical removal and monitoring of oxide impurities in lead lithium using LiCl-KCl melt – ScienceDirect[J]. Fusion Engineering and Design, 156. |
| [12] Garcia-Cano J , Gomis A , Font A , et al. Effect of temperature on the phase-separation ability of KCl in aqueous two-phase systems composed of propanols: Determination of the critical temperature and extension of the results to other salts[J]. Journal of Chemical Thermodynamics, 2019. |
| [13] Reddy L , Sattari M , Davis C J , et al. Influence of KCl and HCl on a Laser Clad FeCrAl Alloy: In-Situ SEM and Controlled Environment High Temperature Corrosion[J]. 2018. |
| [14] [, Erwei, Leng, et al. Effects of KCl and CaCl2 on the evolution of anhydro sugars in reaction intermediates during cellulose fast pyrolysis[J]. Fuel, 2019. |
| [15] Guoliang, Wang, Peter, et al. Potassium capture by coal fly ash: K2CO3, KCl and K2SO4 – ScienceDirect[J]. Fuel Processing Technology, 194:106115-106115. |
| [16] Liu Y C , Liu Y L , Zhao Y , et al. A simple and effective separation of UO2 and Ln2O3 assisted by NH4Cl in LiCl–KCl eutectic[J]. Journal of Nuclear Materials, 2020, 532:152049-. |
| [17] Xsa C , Lei W A , Jg B , et al. Effects of inhibitor KCl on shale expansibility and mechanical properties[J]. Petroleum, 2019, 5( 4):407-412. |
| [18] Xlab C , Na L , Wlab C , et al. Unrevealing the thermophysical properties and microstructural evolution of MgCl 2 –NaCl–KCl eutectic: FPMD simulations and experimental measurements[J]. Solar Energy Materials and Solar Cells, 210. |
| [19] Cui R Z . Solubility measurement and prediction of phase Equilibria in the quaternary system LiCl + NaCl + KCl + H 2 O and ternary subsystem LiCl + NaCl + H 2 O at 288.15 K[J]. Chinese Journal of Chemical Engineering, 2020. |
| [20] Jeong G Y , Sohn S , Y Jeon, et al. Understanding of electrochemical behaviors of niobium in molten LiCl–KCl eutectic for pyrochemical decontamination process[J]. Journal of Nuclear Materials, 2019, 524:39-53. |
| [21] Maji S , Kumar S , Sundararajan K . Exploring LIBS for simultaneous estimation of Sr, Ba and La in LiCl-KCl salt[J]. Optik, 2019, 207. |
| [22] Ab A , Es A , Ss B . The band offset, Half-metallic and optical behavior in the CrSb/KCl [001] interface: By DFT calculation[J]. Chemical Physics Letters, 2019, 714:53-60. |
| [23] Song D H , Ham Y K , Ha J H , et al. Impacts of pre-rigor salting with KCl on technological properties of ground chicken breast – ScienceDirect[J]. Poultry Science, 2020, 99( 1):597-603. |
| [24] Jang P N , Li H M , Wen J K , et al. Electrolytic preparation of Mg-Al-La alloys in KCl-MgCl2-AlF3 molten salts[J]. Journal of Materials Research and Technology, 2019, 8(6). |
| [25] Galvo A C , YP Jiménez, Justel F J , et al. Salting-out precipitation of NaCl, KCl and NH4Cl in mixtures of water and methanol described by the modified Pitzer model[J]. The Journal of Chemical Thermodynamics, 2020:106202. |
| [26] Ww A , Yz A , Hao W B , et al. Optical measurements of KOH, KCl and K for quantitative K-Cl chemistry in thermochemical conversion processes[J]. Fuel, 271. |
| [27] R Núez-González, Aceves R , Cabellos J L , et al. Effect of Substitutional Cu atoms on the Electronic and Optical Properties of KCl: A DFT Approach[J]. Materials Today Communications, 2019, 22:100831. |
| [28] Composition-dependent microstructure evolution in liquid MgCl 2 -KCl: A first-principles molecular dynamics study |
| [29] Poisoning effects of KCl and As 2 O 3 on selective catalytic reduction of NO with NH 3 over Mn-Ce/AC catalysts at low temperature |


Leave a Reply